SEASONAL GROWTH DYNAMICS AND OVERWINTERING OF THE AQUATIC CARNIVOROUS PLANT ALDROVANDA VESICULOSA AT EXPERIMENTAL FIELD SITES
Folia Geobotanica 34: 287-297 (1999)
Lubomír Adamec
Institute of Botany, Academy of Sciences of the Czech Republic, Section of Plant Ecology, Dukelská 135, CZ-379 82 Třeboň, Czech Republic; tel. +420 333 721156, fax +420 333 721136; E-mail adamec@butbn.cas.cz
Keywords: Czech Republic, Growth analysis, Shallow dystrophic habitats, Turion overwintering, Water chemistry
Abstract: New potential sites for the critically-endangered, aquatic carnivorous plant Aldrovanda vesiculosa L. (Droseraceae) were selected in North and South Bohemia (Czech Republic) and both its seasonal growth dynamics and overwintering rate were investigated. Groups of either 5 or 10 plants were planted in 1 x 1 m nylon enclosures in selected shallow, dystrophic waters at the end of May. Plant growth characteristics and water chemistry were investigated at 2 to 5 week intervals over the 1994 growing season inside ten enclosures placed at six sites. Within seven enclosures at three sites, the seasonal growth was very fast and 38-141 turions developed from the initial five plants. Water at these sites was mesotrophic with a high concentration of CO2 above 0.1 mmol.l-1 and pH between 6.2 and 7.6. At the other three sites, plant growth was very poor. The fastest plant propagation was found between late June and mid-August and corresponded with the warmest seasonal period. During this period, the doubling time of the total number of shoot apices was 16.4-34.9 days. Turions developed in mid-September and sank to the bottom by mid-October. In some enclosures, the turions overwintered on the wet bottom whereas they were submersed in the other ones. Though the turions were subject to frosts of up to -20 °C, none died due to the frosts. Grazing of turions by ducks or small rodents was found at some sites. The overwintering rate of turions at sites varied from 0 to 70 % and was not related to seasonal growth rate. Most turions floated to the surface and germinated during late April-early May. It is suggested that considerable turion losses in stable, natural Aldrovanda stands are compensated for by fast seasonal shoot growth and branching which leads to the recovery of an abundant plant population.
INTRODUCTION
Aldrovanda vesiculosa L. (Droseraceae) is a critically-endangered aquatic carnivorous plant, rapidly vanishing from Europe. Its recent European distribution is confined to a few dozens of sites and the number of its potential sites suitable for further spreading has also been reduced markedly (ADAMEC 1995a, KAMIŃSKI et al. 1996). Its rapid decline in Europe in this century has mainly been caused by the eutrophication of waters, water drainage and filling in of water bodies (WALTERS 1979, STUDNIČKA 1984, ADAMEC 1995a). At many sites, the reasons have not yet been elucidated. Aldrovanda has the same life form as the aquatic Utricularia species: it is a rootless, free-floating, submersed plant with fast apical growth (about 1-2 new leaf whorls a day; MAZRIMAS 1978) while the basal part is continuously subjected to senescence and decomposition. In autumn, the plants form apical winter buds (turions) which sink to the lake bottom. Contrary to a seed bank, Aldrovanda turions can only survive from one season to another, not longer (ADAMEC 1999). In Europe, flowering is extremely rare and the plants only reproduce vegetatively by apical branching (BERTA 1961, ADAMEC 1995a).
Aldrovanda occurs irregularly in shallow standing dystrophic waters: lakes, bog and fen pools, backwater pools, and peaty fishponds. In these habitats, it grows within loose and species-poor plant communities and is highly sensitive to being overgrown by filamentous algae, and to competition with any other aquatic plants that form dense stands (MAZRIMAS 1978, STUDNIČKA 1984). Aldrovanda is considered to be strictly stenotopic (STUDNIČKA 1984), but its ecological requirements have not yet been sufficiently explained and the same holds for its growth strategy (BERTA 1961, STUDNIČKA 1984, KAMIŃSKI 1987a,b, ADAMEC 1995a, 1997a). As recently reviewed by ADAMEC (1995a), Aldrovanda is tolerant of some ecological factors (e.g, total alkalinity, concentration of mineral nutrients, irradiance) while being rather intolerant of others (high CO2 concentration, humic acids, high temperature, sufficient prey, etc.). Due to its extreme rarity and the remoteness of its sites, its growth dynamics have never been studied and only static population investigations including plant flowering, turion formation and sinking were carried out (AFANAS'EV 1953, BERTA 1961, KOMIYA 1966, STUDNIČKA 1984, KAMIŃSKI 1987a,b, AKERET 1993). Preliminary data on the natural overwintering of Aldrovanda turions show that only a small proportion of turions (ca. 0-30%) survive the winter (KAMIŃSKI & ADAMEC, unpubl. data). These figures would indicate a high risk for the survival of populations.
The aim of this study was to investigate the seasonal growth dynamics including both summer growth and overwintering of Aldrovanda plants introduced within nylon enclosures under natural conditions. The sites were selected to correspond to ecological optimum of this species. Thus, the aim was to test the selection of these sites by a growth experiment in the field. Physical and chemical habitat factors were also measured at the sites. Using the results of this study, Aldrovanda has been introduced into some suitable sites (ADAMEC & LEV 1999).
MATERIALS AND METHODS
Aldrovanda vesiculosa plants used for growth experiments were cultivated outdoors in a 1 m2 plastic container as described by ADAMEC (1997b). Aldrovanda plants used for cultivation were collected in Lake Długie in east Poland (51°26'N, 23°06'E).
During the season of 1994, the introduced plants of Aldrovanda were grown in nylon enclosures in selected shallow dystrophic waters in the Czech Republic. These sites resembled the natural ones in Poland as to their water depth, the character of dominant emergent vegetation and degree of shading by it, water transparency, and CO2 concentration (ADAMEC 1997a). The selection of the sites and actual siting of the enclosures was based on assessments of suitable water depth (0.15-0.5 m), water level fluctuations (minimum summer level > 2 cm), the character of dominant aquatic and emergent vegetation (reed or sedge stands), degree of shading by emergent vegetation (> 20% of incident radiation), water transparency (as high as possible), and CO2 concentration (> 0.1 mmol.l-1). At these sites, 1-3 nylon enclosures were placed in different types of vegetation. The bottomless and topless enclosures of 1 x 1 m were made of a band of nylon mesh (mesh diameter 1.5 mm) 33 or 50 cm wide. They were tightly attached to the bottom and extended about 5 cm above the water surface. In this way, the plants were prevented from escaping, but their growth was not limited in any way. In total, 10 enclosures were placed in 6 sites, two in south Bohemia (Třeboňsko Biosphere Reserve) and four in north Bohemia (Česká Lípa District).
Description of sites
The Domanínský fishpond lies 3 km SW of Třeboň, S Bohemia (48°59' N, 14°44' E; altitude 446 m a.s.l.). It is a eutrophic fishpond with an irregular water inflow. The fishpond is regularly emptied at the end of October-early November and its normal water level is again reached between February-April. Three 50 cm enclosures were placed in a shallow wetland in the northern tip of the pond:
D1, in a loose reed stand, at a water depth of 30-40 cm. Plant dominants (> 40% of total coverage): Phragmites australis, aquatic moss Calliergon cordifolium; subdominant (10-40% of total coverage): Carex gracilis. D1 was situated about 12 m from D2 and D3.
D2, in a sedge stand, at a water depth of 25-35 cm. Dominant: Carex rostrata; subdominant: Utricularia australis.
D3, in a dense reed stand, at a water depth of 25-35 cm. Dominants: Phragmites australis, Calliergon cordifolium (100% coverage).
The Ptačí blato fishpond lies 12 km NW of Třeboň (49°05' N, 14°41' E; 434 m a.s.l.) and is an eutrophic fishpond with an irregular water inflow. The fishpond is regularly emptied at 2-year intervals, at the end of October-early November and its normal water level is again reached between April-May. It was emptied in early November 1994. Along its whole SE shoreline, many shallow dystrophic pools of 0.04-0.1 ha in size have been excavated in the fen soil. Two 33 cm enclosures were placed in two of these pools:
PB1, in the second pool from the north in a sedge stand, with a water depth of 15-25 cm. Dominants: Carex rostrata and the aquatic moss Drepanocladus aduncus; subdominants: Utricularia minor and U. ochroleuca.
PB2, in the ninth pool in a loose reed stand, at a water depth of 15-25 cm. Dominants: Phragmites australis, Drepanocladus aduncus, Calliergon cordifolium; subdominant: Utricularia minor.
The Mariánský fishpond lies 2 km south of Doksy, N Bohemia (50°33' N, 14°41' E; 275 m a.s.l.). It is a shallow dystrophic abandoned fishpond which is never emptied. Two 50 cm enclosures were placed in the southern part of the reed belt, only about 3 m from each other:
M1, in a loose reed stand, at a water depth of 30-45 cm. Dominant: Phragmites australis; subdominant: Myriophyllum verticillatum.
M2, in a dense reed stand, at a water depth of 30-40 cm. Dominant: Phragmites australis.
The Poselský fishpond lies 1.5 km south of Doksy (50°33' N, 14°41' E; 274 m a.s.l.). It is a mesotrophic fishpond with extensive reed stands. One 33 cm enclosure was established in a small shallow pool at the boundary between the reed stand and a forest on peaty soil on the SE shore:
PO, in a loose reed stand, at a water depth of 10-15 cm. Dominant: Phragmites australis; subdominants: Utricularia minor, Eriophorum vaginatum.
The Jestřebí fen pool lies 1 km SE of Jestřebí, N Bohemia (50°36' N, 14°37' E; 260 m a.s.l.). It is a small fen pool (20 m2) in an excavated fen. One 33 cm enclosure was placed in the pool:
JE, in a loose reed stand, at a water depth of 15-25 cm. Dominants: Phragmites australis, Utricularia minor, Chara fragilis, Spirogyra sp.
The Srní Potok pool lies 2 km north of Mimoň, N Bohemia (50°41' N, 14°44' E; 280 m a.s.l.) and is a shallow wetland in the Ploučnice river floodplain. One 33 cm enclosure was placed in a shallow backwater pool (ca. 0.04 ha) between Carex gracilis tussocks:
SP, in a dense stand of submersed macrophytes, at a water depth of 20-25 cm. Dominants: Myriophyllum verticillatum, Potamogeton berchtoldii, Utricularia australis, Callitriche hamulata.
Table 1. Water chemistry and mean level of shading on the water surface (PAR; % of incident irradiance in the open area) at experimental sites of Aldrovanda in nylon enclosures in the Czech Republic in July 1994. Mean of two samples from the beginning and end of July (the difference usually < 10%) is shown. The CO2 concentration is based on both calculated and measured values. HAT - sum of concentrations of humic acids and tannins; TA - total alkalinity.
|
Site |
NO3- |
NH4+ |
PO4 |
|
|||||||||
|
(N) |
(N) |
(P) |
K |
Na |
Ca |
Mg |
HAT |
O2 |
pH |
TA |
CO2 |
PAR |
|
|
microg.l-1 |
mg.l-1 |
|
mmol.l-1 |
% |
|||||||||
|
D1 |
0 |
17 |
9 |
14.5 |
8.0 |
31.0 |
6.7 |
6.7 |
4.0 |
7.23 |
2.87 |
0.38 |
47 |
|
D2 |
25 |
21 |
14 |
10.2 |
7.4 |
19.1 |
6.7 |
7.2 |
1.8 |
7.02 |
2.11 |
0.34 |
49 |
|
D3 |
0 |
37 |
12 |
16.4 |
8.6 |
34.6 |
6.7 |
7.3 |
9.8 |
7.61 |
3.09 |
0.15 |
18 |
|
PB1 |
2 |
25 |
18 |
8.1 |
10.2 |
37.8 |
6.0 |
28.3 |
6.9 |
6.87 |
1.79 |
0.52 |
54 |
|
PB2 |
0 |
25 |
14 |
0.6 |
7.0 |
32.0 |
4.5 |
36.0 |
3.3 |
6.46 |
1.02 |
0.86 |
47 |
|
Ml |
0 |
17 |
11 |
1.1 |
5.6 |
10.5 |
2.4 |
3.1 |
6.9 |
6.22 |
0.26 |
0.42 |
62 |
|
M2 |
0 |
28 |
13 |
0.9 |
5.4 |
11.1 |
2.3 |
3.5 |
9.3 |
6.51 |
0.34 |
0.32 |
32 |
|
PO |
131 |
122 |
11 |
2.5 |
4.5 |
7.0 |
2.1 |
1.5 |
9.0 |
4.23 |
0.00 |
0.24 |
68 |
|
JE |
6 |
33 |
22 |
3.9 |
12.4 |
32.9 |
6.1 |
8.4 |
15.3 |
8.58 |
2.33 |
0.02 |
40 |
|
SP |
2 |
50 |
34 |
5.4 |
13.4 |
31.2 |
6.0 |
14.8 |
7.2 |
7.01 |
1.93 |
0.49 |
83 |
Between 21-29 May 1994, five adult Aldrovanda plants cultivated at Třeboň were introduced to each of the nylon enclosures. In two cases (JE and SP), ten plants were introduced. The non-branched plants were 6.0 cm long, with 10-14 whorls of mature leaves. Growth characteristics of the introduced populations, water level, and water chemistry in the enclosures were investigated for the whole 1994 season at 2-5 week intervals. The following variables were measured on each plant: total length of main shoot, number of leaf whorls with mature traps, number of first and second order branches, number of internodes between two successive branches, and number of flowering plants and mature capsules. On 17-20 September 1994, turions were counted on each plant. Branched turions with non-separated apices were counted as one while those separated distinctly from each other counted as two. Possible injuries on the overwintering turions that were visible on the bottom sediment, and the water level were checked in all enclosures in S Bohemia over the winter at 1-2 month intervals. Overwintered turions were counted in all enclosures at five intervals between 20 April and 11 August 1995. Only those turions and/or germinating plants which floated close to the water surface were counted and removed from the enclosures, while the remaining were inactive or dead. The proportion of germinating and branched turions was also estimated to assess better the phenological stages and propagation capacity of the plants.
The dissolved O2 concentration, pH, and water temperature were measured in the enclosures in the zone surrounding Aldrovanda shoots, 2 cm below the water surface, between 10:00-18:00 local summer tíme (for the details see ADAMEC 1997a). Occasionally, a Severingshausen-type CO2 sensor (Labio, Prague, Czech Republic) was used to directly measure the free CO2 concentration. As sensor response was very slow in the unstirred medium (ca. 30 min), the sensor was allowed to float on a small polystyrene block with the tip of the sensor in the vicinity of the plants for 40-100 min, until its stable signal was recorded. Since some variation of pH (+ 0.10) usually occurred in various parts of the stand within the enclosure, pH was measured close to the CO2 sensor and mean pH values taken. At the beginning of July, an estimate of the level of shading by emergent vegetation was made using a PAR sensor (400-700 nm; ADAMEC 1997a). The results are expressed as percentage incident PAR penetrating the water surface.
Table 2. Aldrovanda seasonal growth and overwintering in nylon enclosures at the experimental sites. Plants were introduced (number of plants in brackets) between 21-29 May 1994. Numbers of individual plants and all apices estimated on 12-17 August, numbers of formed turions on 17-20 September 1994. Turion overwintering was evaluated in the 1995 season in the given intervals and is expressed as cumulative numbers.
|
|
Seasonal growth in 1994 |
Turion overwintering in 1995 |
||||||
|
Site |
12-17 Aug |
17-20 Sep turions |
20-30 Apr |
6-7 May |
31 May- -3 Jun |
12 Jun- -13 Jul |
2-11 Aug |
|
|
plants |
apices |
|||||||
|
D1 (5) |
30 |
41 |
50 |
18 |
26 |
34 |
34 |
35 |
|
D2 (5) |
23 |
31 |
45 |
7 |
13 |
15 |
15 |
15 |
|
D3 (5) |
18 |
31 |
38 |
3 |
8 |
8 |
8 |
8 |
|
PB1 (5) |
51 |
107 |
141 |
6 |
38 |
38 |
38 |
39 |
|
PB2 (5) |
56 |
72 |
85 |
1 |
6 |
16 |
27 |
36 |
|
M1 (5) |
67 |
173 |
110 |
0 |
- |
4 |
4 |
4 |
|
M2 (5) |
48 |
146 |
95 |
3 |
- |
11 |
11 |
12 |
|
PO (10) |
20 |
20 |
15 |
0 |
- |
0 |
0 |
0 |
|
JE (10) |
16 |
18 |
4 |
2 |
- |
2 |
2 |
2 |
|
SP (5) |
10 |
12 |
6 |
0 |
- |
0 |
0 |
0 |
Total alkalinity (TA; TA=[HCO3-]+2.[CO32-]+[OH-]-[H+]) was estimated by the Gran titration (TALLING 1973) of filtrated water samples. The concentration of CO2 was calculated from TA and pH (HELDER 1988). At the beginning and end of July, filtrated water samples were collected from the surface and analyzed for macro-nutrients. NO3--N, NH4+-N, and PO4-P concentrations were estimated using a FIAstar 5010 Analyzer (Tecator, Sweden) and K+, Na+, Ca2+, and Mg2+ using an atomic absorption spectrophotometer (AAS 1N, Zeiss, Germany). At the beginning of July, the concentration of humic acids in the filtrated water samples was estimated by a simple colorimetric method (PEKÁRKOVÁ & LISCHKE 1974). The method is based on the extraction of humic acids from an acidified sample to amyl-alcohol and on their subsequent dissolving in the alkaline aqueous phase. Optical density was measured at 420 nm. It was found that the method was also very sensitive to tannin and thus it records a sum of humic acids and tannins.
RESULTS
Chemical and physical factors
The main form of mineral N in water in the enclosures was NH4+ (Tab. 1 ) but the NH4+-N concentrations at the sites with vigorous growth of Aldrovanda (D1-3; PB1, 2; M1, 2; see Tab. 2) never exceeded 40 microg.l-1. At these sites, PO4-P concentrations were within the narrow range of 9-18 microg.l-1. K concentration was as low as 0.3-0.7 mg.l-1 at PB2 and M1, 2. The selected sites differed greatly in their concentrations of humic acids plus tannins. O2 concentration in water always reached 15% of the saturation concentration. However, anoxia was recorded in the upper 1 cm layer in bottom sediments of all enclosures at the end of July (data not shown). pH varied between 4.2 (PO) and 9.3 (JE) at sites in July. Except for D3, JE, and SP, pH was rather stable. Mean pH values reached 6.2-7.6 at favourable sites (cf. Tab. 2), at which CO2 concentration was also high at the peak of the growing season (July-August): always above 0.1 mmol.l-1, usually between 0.2-0.6 mmol.l-1. TA values differed greatly among sites, from zero (PO) to 3 meq.l-1 (D1, 3). Light conditions were favourable for the fast growth of Aldrovanda in all enclosures except D3 where it was only about 18% of full daylight.
The seasonal growth and overwintering
The seasonal growth of Aldrovanda was very vigorous in all enclosures at the Domanínský, Ptačí blato, and Mariánský fishpond sites (Tab. 2). At these sites, the initial five non-branched plants propagated so fast that the total number of apices was 31-173 in mid-August (i.e. 2-3 weeks before the end of the growing season) and 38-141 turions were formed in September.

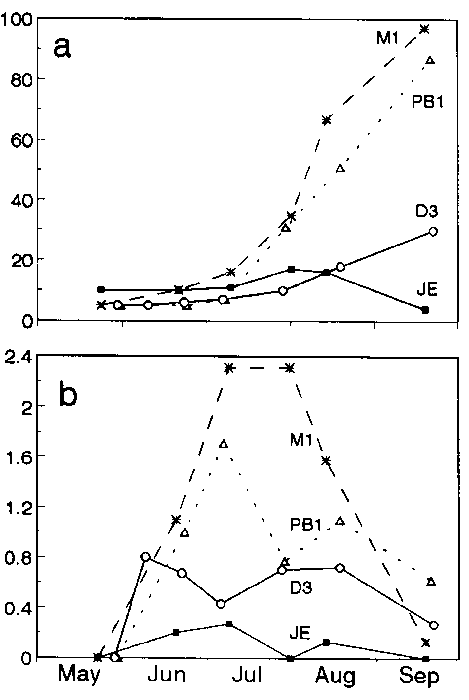
Fig. 1. Seasonal growth dynamics of Aldrovanda vesiculosa in enclosures. Four typical examples are shown. a - number of plants; b - all branches per plant.
At all favourable sites and at JE, plant growth was most rapid between the end of June and mid-August (for the typical examples see Fig. 1). The period of fastest growth roughly corresponded with the extremely long warm period of the 1994 summer (23 June-9 August) when maximum daily air temperatures were usually above 26-28 °C. At favourable sites, the total number of plants and apices approximately followed an exponential curve (Fig. 1) but the curve's sigmoidal character was distinct at only D1, 3 and PB1, 2. Plant growth had slowed down before the turions were formed in mid-September (Tab. 3). Seasonal growth characteristics were about the same in the groups of adjoining enclosures (D1 and D2; M1 and M2) but also at PB1 and PB2.
Tab. 3. Doubling time of the total number of apices of Aldrovanda plants growing at the sites with vigorous growth.
|
Time interval |
Doubling time (days) at |
||||||
|
D1 |
D2 |
D3 |
PB1 |
PB2 |
M1 |
M2 |
|
|
19-22 Jun to 5-8 Jul |
12.9 |
15.1 |
∞ |
15.1 |
14.8 |
14.2 |
16.1 |
|
5-8 Jul to 27-30 Jul |
23.0 |
79.8 |
28.7 |
14.3 |
21.4 |
19.5 |
13.8 |
|
27-30 Jul to 12-17 Aug |
67.1 |
48.8 |
24.2 |
20.8 |
51.5 |
22.5 |
25.8 |
|
19-22 Jun to 12-17 Aug |
24.1 |
34.9 |
34.9 |
16.4 |
23.7 |
17.7 |
16.4 |
The branching of main and daughter shoots was fairly regular. On the main shoots, an average of 6.17 internodes (n=159; median 6.0; range 3-11; 85.5% of all values lay within 5-7) occurred between two successive branches. Daughter shoots only started forming visible branches at a stage of 13.6 mature leaf whorls (n=22; median 13.5; range 8-19), when attached to main shoots, and at ca. 11-14 whorls when separated. Plant length correlated closely with the number of mature leaf whorls (data not shown). At all sites, plants were longest in July, with the longest plants occurring at M1 and M2 (mean 16.7-23.3 cm, maximum 43.0 cm) whereas those at PO were the shortest (mean 2.2-2.9 cm, maximum 3.6 cm). Plants only flowered at D1 (3 plants with 7 flowers) and D2 (4 plants with 7 flowers and 1 ripe capsule with 1 seed), from late July to mid-September.
Both the percentage of overwintered turions, and the dynamics of their floating up, differed markedly between individual sites but also within individual enclosures at certain sites (Tab. 2). The turions overwintered best at D1 and JE (70 and 50%), while only 4-13% overwintered at M1 and M2, and none at PO and SP. At the other sites, between 21 to 42% of the produced turions overwintered. In total, the largest number of turions (35-39) overwintered in the D1, PB1 and PB2 enclosures. Turions branched at a mean of 0.55 branches per turion. Turions started floating up in mid-April and the overwhelming majority (92-100%) floated up in all enclosures, except for PB2 (44%), by 31 May-3 June. All the turions counted as having overwintered in the enclosures were able to germinate in the outdoor culture. Overall, the rapid seasonal propagation of Aldrovanda in the enclosures did not correlate with the high turion overwintering rate (n=7; correlation coefficient 0.011).
DISCUSSION
Habitat characteristics and plant growth
Six sites with 10 microsites were selected in the Czech Republic to verify their suitability for the growth of A. vesiculosa. At sites favourable to its seasonal growth (Tab. 2) and also at the JE site, water was relatively poor in main mineral nutrients (N, P, and sometimes K) and may be characterized as mesotrophic and dystrophic (Tab. 1). A similar level of NO3-, NH4+, HPO42-, and K+ was found at some natural sites of Aldrovanda in Japan (KOMIYA 1966), Slovakia (STUDNIČKA 1984), Switzerland (AKERET 1993), and Poland (KAMIŃSKI, unpubl. data). However, growth experiments show that Aldrovanda gains the majority of its mineral nutrients from prey (KAMIŃSKI 1987b). Growth was obviously not limited by prey availability as at least one-third of all traps usually had some prey. The same was found by AKERET (1993) and STUDNIČKA (1984) at suitable sites. In enclosures with the faster seasonal growth (D1-3; PB1, 2; M1, 2), pH values ranged within only 6.2-7.9 over July-August. As the pH between 6.3 to 7.5 was also recorded at many prolific natural sites of Aldrovanda (KOMIYA 1966, STUDNIČKA 1984, AKERET 1993, ADAMEC 1997a) this range possibly represents its ecological optimum. However, it also grew rapidly in a Sphagnum-dominated pool at pH 4.6-4.8 (ADAMEC, unpubl. data) and growth in a mineral medium was best at the pH 3.5-5.5 (KAMIŃSKI 1987a). Thus, it is also an acid-tolerant species.
A typical feature at all favourable sites was a relatively high CO2 concentration which never fell below 0.1 mmol.l-1 during July-August, and was usually between 0.2-0.6 mmol.l-1 (Tab. 1 ). This range was also found at most of the Polish sites, where CO2 never fell below 0.14 mmol.l-1 (ADAMEC 1997a), as well as at other sites (KOMIYA 1966, AKERET 1993). High CO2 concentrations at selected sites represented one of the necessary conditions for its vigorous growth. Aldrovanda is a strict photosynthetic CO2 user and a reduction of CO2 concentration below ca. 0.15 mmol.l-1 leads to a steep decrease in its photosynthetic rate (ADAMEC 1997a). The effect of carbon utilization from prey on its growth was clearly demonstrated at JE. Here, pH was 9.2 + 0.1 at the end of July and CO2 concentration (ca. 3.3 micromol.l-1) was obviously below the CO2 compensation point of photosynthesis (5.9-8.0 micromol.l-1; ADAMEC 1997a). Thus, with a CO2 shortage, a great deal of carbon is taken up from trapped prey. The plants were relatively weak, with small traps, and did not branch at all (Fig. lb).
In accordance with data from Poland, about 20% of incident PAR might represent a lower optimum light limit and any higher irradiance up to full sun is favourable (Tab. 1; cf. ADAMEC 1997a). However, the photosynthesis of Aldrovanda confirmed its very photo- and thermophilous character (ADAMEC 1997a). Over the warm and dry summer period, the afternoon temperature at the water surface on sampling days ranged within 23-29 °C at favourable sites (data not shown). The water level in all enclosures was falling during the same warm period and reached a minimum of 1-4 cm in all D and PB enclosures in August, while at least 5-8 cm remained in the others. As a result of insufficient water depth, plant growth was slowed down (intraspecific competition) at D1 and PB2 in early August. To maintain fast growth, the water level at Aldrovanda sites should not fall below 5 cm during the growing season.
Present data suggest that a 10 cm deep fibrous sediment consisting of partly decomposed litter of emergent vegetation and dead mosses can form a suitable aquatic medium for Aldrovanda growth. At the selected sites, loose Phragmites australis (D1; PB2, M1, M2) and Carex rostrata dominated stands (D2, PB1) appeared to be the most favourable habitats for its growth. As reviewed by ADAMEC (1995a), these habitats represent its ecological optimum.
The enclosures used prevented plants from moving to other microsites but they did not influence other abiotic and biotic factors. The movement of plants by wind within a site may be an important ecological factor, as a good proportion of their population may drift to microsites with unfavourable growth conditions and die (ADAMEC & LEV 1999).
Seasonal growth dynamics
Under favourable growth conditions, the main shoot apex can form about one leaf whorl a day (KOMIYA 1966, MAZRIMAS 1978). Thus, each main shoot apex can branch on average every 6 days. However, the growth rate of young daughter branches (below 6 whorls) is only about 67% of that above, and is only the same later on. Thus, daughter shoots may branch after about 16.6 days on average. This period agrees with the measured doubling time of apices (Tab. 3). A similar doubling time of biomass of ca. 20 days can be deduced for plants in Japan (KOMIYA 1966). Since European Aldrovanda populations propagate only vegetatively by the apical branching of shoots (ADAMEC 1995a), the frequency of branching is the principal growth parameter to be used as a criterion for determining the suitability of growth conditions (KAMIŃSKI 1987a) and the propagation rate (KOMIYA 1966). At three sites over the 1994 growing season, the total number of apices rose 7.6-34 times (Tab. 2) and plants were richly branched (0.3-2.3 branches per plant; Fig. l). Plants at 10 Polish sites were also fairly branched (0.3-1.5 branches per plant; KAMIŃSKI 1987a) and their density was relatively high in early August (8-341 plants.m-2).
It may be assumed that plants at natural sites also propagate many times over the season. However, plants freely introduced to PB1 and PB2 propagated very poorly (1.0-1.4 times) in the warm and dry 1995 season, whereas propagation was very rapid (14-36 times) in the colder and rainy 1996 season (ADAMEC & LEV 1999). Thus, the water level mainly determines the seasonal growth dynamics of Aldrovanda at natural sites and, also, explains why plant abundance and density are highly variable between seasons (AFANAS’EV 1953, KAMIŃSKI 1987a). Moreover, the gradual decline of actual water depth in its stands, due to either the summer fall in water level or to an accelerated accumulation of plant litter, may be suggested as one of the reasons for Aldrovanda decline at natural sites.
All the selected sites in S Bohemia (D1-3; PB1, PB2) exhibit marked fluctuations of water level in the summer seasons, with only 1-4 cm left in dry seasons while ca. 50 cm in rainy ones. Moreover, some sites (PB1, PB2, JE, SP) have been undergoing gradual eutrophication since 1994-1996 (ADAMEC &. LEV 1999). Thus, the ecological character of most of the sites selected is highly variable in time, partly also due to water management in adjacent eutrophic fishponds. Overall, the presented results of a one-season growth experiment (Tab. 2) should be interpreted cautiously concerning the long time survival of stable Aldrovanda subpopulations (cf. ADAMEC & LEV 1999).
Plant overwintering
At all the experimental sites, apical turions were formed in mid-September as a result of the fall in water temperature. The steep decline of apices (turions) at M1, M2, JE, and SP at the end of the growing season (Tab. 2; Fig. 1) was caused, evidently, by grazing by ducks. Obviously, waterfowl graze on Aldrovanda only when turions, rich in sugars (ADAMEC 1995b), are formed. Thus, herbivorous waterfowl can reduce Aldrovanda populations markedly but to what extent is not known at natural sites. The turions at M1 and M2 were unripe while those at other sites (esp. PB1 and PB2) were much riper and just beginning to break off from aged shoots. This phenological difference between the sites could be caused by different concentrations of humic acids (Tab. 1) as they were found to markedly stimulate turion ripening and subsequent sinking to the bottom (KAMIŃSKI 1987b). By 15 October, nearly all turions had sunk to the bottom at D1-3 and PB1 and PB2. Thus, turions had sunk between late September and mid-October (cf. BERTA 1961). The percentage of overwintered turions was highly variable between different sites but also within different enclosures at individual sites, in contrast with fairly consistent seasonal plant growth (Tab. 2).
After turions had already sunk in October, the majority of them became immersed in bottom sediment and were not visible. At D1-3 and PB1, PB2, a small number of turions lay on the very wet bottom over the winter. These turions were particularly subjected to winter frosts (down to ca. -20 °C at the turion level), however, no dead turions damaged by frosts were found. Though Aldrovanda turions were killed by -10 or -12 °C in a refrigerator (KAMIŃSKI 1987b) they fully withstood the same frosts when stored outdoors on a wet bottom and were adapted to them (ADAMEC 1995b). Over the winter season, some turions lying on the wet bottom at PB1 and PB2 were seriously damaged by grazing by small rodents, and overall many turions were grazed. Thus, turion overwintering above water is "risky" due to grazing by herbivores. At PB1, PB2 and D2, the turions overwintered much better under water (35-100%; ADAMEC & LEV 1999; cf. Tab. 2).
Turions first started floating up at D1, D2 and PB1 in early April, while a good proportion of them floated up in most enclosures in late April (Tab. 2) after upper bottom temperatures had reached ca. 12-15 °C (data not shown). Almost all live turions had floated up by 31 May-3 June (cf. AFANAS'EV 1953). At PB2, however, 56% of all overwintered turions were released as late as mid-June to mid-August due to the passing of roe deer and to the burial of the turions.
It is not known whether the turions also require light in order to float up (cf. NEWTON et al. 1978, BEER 1985). The majority of turions overwinter covered with a thin layer of organic sediment, under conditions of deep shade, or darkness, and anoxia. It is probable that the optical and mechanical properties of this covering can be a key factor for turion survival. Over the winter, the sedimentation rate of litter is high in shallow Carex and Phragmites stands, and the turions are additionally covered with new litter which shades them and prevents them from floating up. Therefore, dense emergent stands cannot only limit the seasonal growth of Aldmvanda, but also reduce its overwintering rate. The PB1 and M1 sediments in the refrigerator showed a high turion overwintering rate (80-90%; ADAMEC, unpubl. data). Thus, the low rate found at PB1 (28%) and M1 (4%) would appear to be due to other factors, mainly grazing by rodents (PB1) or ducks (M1). The germination (sprouting) of turions of aquatic plants is light dependent (BARTLEY & SPENCE 1987) and the same was confirmed for Aldrovanda (ADAMEC 1999). Overall, the floating up of Aldrovanda turions is a critical phase of its overwintering as turions cannot germinate when covered with a layer of sediment.
CONCLUSIONS
The suitability of the selected sites for the rapid seasonal growth of Aldrovanda does not need to correspond with the suitability for turion overwintering. At permanent and stable natural sites of Aldrovanda, both parts of its seasonal cycle must take place. Turion overwintering usually leads to considerable population losses and is the most critical phase of its seasonal cycle. The floating up of turions from the bottom is the primary limiting process of turion overwintering, while the grazing of turions is also limiting. Within stable natural populations, these population losses are compensated for by fast apical shoot growth and branching which leads to fast plant propagation, up to the level of a carrying capacity for a stand. Such an r-type growth strategy fully exploits the relatively short growing season of ca. 100-120 days (cf. WINSTON & GORHAM 1979, FRIDAY 1989, for Utricularia vulgaris).
Loose Phragmites australis and Carex rostrata dominated shallow stands with very loose aquatic vegetation represent the ecological optimum for Aldrovanda growth. As the important ecological requirements of Aldrovanda are combined, this species may be characterized as stenotopic although it is tolerant of many factors alone. Yet, present data indicate that favourable sites may also exist in shallow dystrophic wetlands close to highly eutrophicated fishponds in an intensively agricultural landscape. The chances for its survival will rely on the maintenance and protection of all existing natural sites and on the selection of potential new sites, even in such regions where it has not occurred in the past.
Acknowledgements: The paper is dedicated to my colleague Dr. Jan Květ on the occasion of his 65th birthday and for his whole life study of wetland plant ecology and great merits in wetland protection. This research was supported by the Grant Agency of the Academy of Sciences of the Czech Republic (project No. 605401). The author is grateful to Dr. R. Kamiński (Poland) for providing some unpublished data, Dr. L. Klimeš (Třeboň, Czech Republic) for critically reading the manuscript, and to Mr. S. Ridgill (U.K.) for correction of the language.
REFERENCES
ADAMEC L. (1995a): Ecological requirements and recent European distribution of the aquatic carnivorous plant Aldrovanda vesiculosa L. - A review. Folia Geobot. Phytotax. 30: 53-61.
ADAMEC L. (1995b): Ecophysiological study of the aquatic carnivorous plant Aldrovanda vesiculosa L. Acta Bot. Gallica 142: 681-684.
ADAMEC L. (1997a): Photosynthetic characteristics of the aquatic carnivorous plant Aldrovanda vesiculosa. Aquatic Bot. 59: 297-306.
ADAMEC L. (1997b): How to grow Aldrovanda vesiculosa outdoors. Carniv Pl. Newslett. 26: 85-88.
ADAMEC L. (1999): Turion overwintering of aquatic carnivorous plants. Carniv Pl. Newslett. 28: 19-24.
ADAMEC L. & LEV J. (1999): The introduction of the aquatic carnivorous plant Aldrovanda vesiculosa to new potential sites in the Czech Republic: A five-year investigation. Folia Geobot. 34: 299-305.
AFANAS’EV K.S. (1953): Novoe mestonachozhdenie Aldrovanda vesiculosa v SSSR (New site of Aldrovanda vesiculosa L. in USSR). Bot. Zhurn. 38: 432-434.
AKERET B. (1993): Ein neuer Fundort von Aldrovanda vesiculosa L. in der Nordschweiz and einige Bemerkungen zu Stratiotes aloides L. Bot. Helv. 103: 193-199.
BARTLEY M.R. & SPENCE D.H.N. (1987): Dormancy and propagation in helophytes and hydrophytes. Arch. Hydrobiol. (Beih.) 27: 139-155.
BEER S. (1985): Effects of CO2 and O2 on the photosynthetic O2 evolution of Spirodela polyrhiza turions. Pl. Physiol. 79: 199-201.
BERTA J. (1961): Beitrag zur Őkologie and Verbreitung von Aldrovanda vesiculosa L. Biológia (Bratislava) 16: 561-573.
FRIDAY L.E. (1989): Rapid turnover of traps in Utricularia vulgaris L. Oecologia 80: 272-277.
HELDER R.J. (1988): A quantitative approach to the inorganic carbon system in aqueous media used in biological research: dilute solutions isolated from the atmosphere. Pl. Cell Environm. 11: 211-230.
KAMIŃSKI R. (1987a): Studies on the ecology of Aldrovanda vesiculosa L. I. Ecological differentiation of A. vesiculosa population under the influence of chemical factors in the habitat. Ekol. Polska 35: 559-590.
KAMIŃSKI R. (1987b): Studies on the ecology of Aldrovarula vesiculosa L. II. Organic substances, physical and biotic factors and the growth and development of A. vesiculosa. Ekol. Polska 35: 591-609.
KAMIŃSKI R., ADAMEC L. & BRECKPOT C. (1996): Report on recent sites of Aldrovanda vesiculosa (Droseraceae) in Poland. Fragm. Florist. Geobot. 41: 291-294.
KOMIYA S. (1966): A report on the natural habitat of Aldrovanda vesiculosa found in Hanyu City. Amatores Herb. 27: 5-13.
MAZRIMAS J.A. (1978): Aldrovanda. Carniv. Pl. Newslett. 7: 102-103.
NEWTON R.J., SHELTON D.R., DISHAROON S. & DUFFEY J.E. (1978): Turion formation and germination in Spirodela polyrhiza. Amer. J. Bot. 65: 421-428.
PEKÁRKOVÁ K. & LISCHKE P. (1974): Chemické metody analýzy vod (Chemical methods of water analyses). SNTL, Prague.
STUDNIČKA M. (1984): Masožravá rostlina aldrovandka měchýřkatá (The carnivorous plant Aldrovanda vesiculosa). Živa 32: 173-176.
TALLING J.F (1973): The application of some electrochemical methods to the measurement of photosynthesis and respiration in fresh waters. Freshwater Biol. 3: 214-243.
WALTERS S.M. (1979): Conservation of the European flora: Aldrovanda vesiculosa L., a documented case-history of a threatened species. In: HEDBERG I. (ed.), Systematic botany, plant utilization and biosphere conservation, Almquist and Wiksell International, Stockholm, pp. 76-82.
WINSTON R.D. & GORHAM P.R. (1979): Turions and dormancy states in Utricularia vulgaris. Canad. J. Bot. 57: 2740-2749.
Received 19 October 1998, revision received 25 February 1999, final revision received 14 September 1999 and accepted 19 September 1999.
Copyright (c) 1995-2002 www.BestCarnivorousPlants.com